Driven by a continued focus on personalised medicine, Cell & Gene therapies have experienced continued growth over the past 15 years, with the number of FDA-approved therapies more than doubling since 2020.
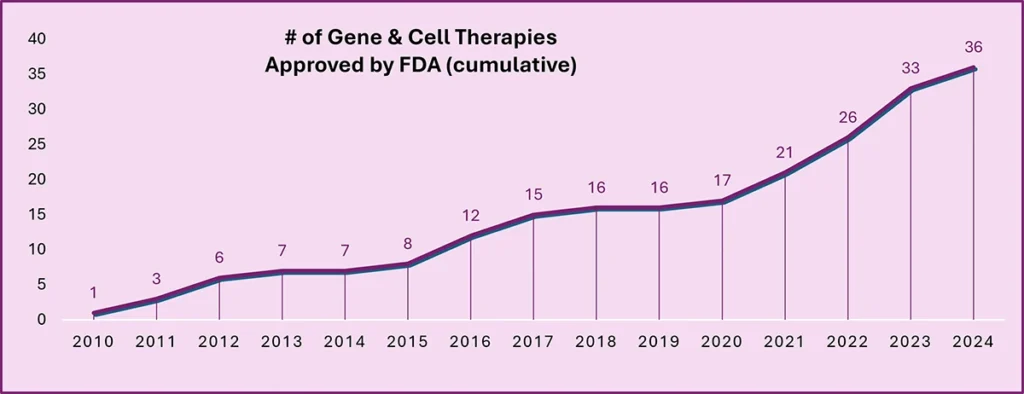
Although the increased availability of Cell & Gene therapies is great news for patients, particularly those with rare diseases or those for which there is no alternative treatment, ensuring patients can access these therapeutics presents significant challenges for governments, regulators & healthcare providers. This then begs the question: Do we have the necessary frameworks and infrastructure to deliver these novel therapeutics to patients?
A report recently published by the Association of the British Pharmaceutical Industry (ABPI) suggests that in the UK, the NHS has coped well to date in terms of providing access to these medicines, although the influx of new approvals for future Cell & Gene therapies presents the following significant challenges:
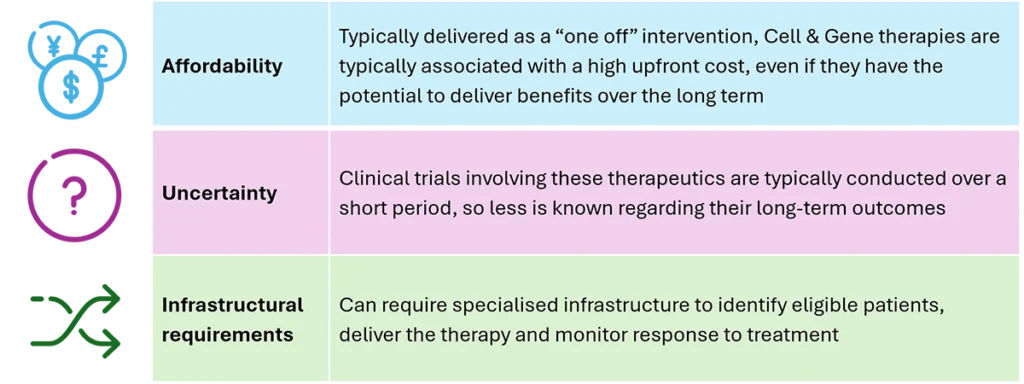
Given the extremely high cost of Cell & Gene Therapies, affordability represents by far the main barrier to providing access to these medicines. Therefore, the main recommendation of the ABPI report, which could also apply to other countries, is to consider innovative pricing models that would aim to spread the cost of these treatments over a longer period, rather than relying on a high initial upfront cost, which could lead them to being judged to be unaffordable for most. In fact, several alternative payment models have already been deployed for Gene & Cell Therapies in several countries, including the US, Europe & Asia. Such innovation could enable other countries (including the UK) to develop policies that could enable greater patient access to these expensive but highly valuable treatments.
The above example is just one of the various challenges and questions that we are dealing with every day for our Life Science clients. By looking at both the driving force itself (in this case personalised medicine) and how that impacts their brand/offer, we can help our clients devise strategies (e.g. pricing, sales, market sizing) to try and reduce the impact of any unintended consequences they cause.
If you have any additional questions or would just like to discuss our Life Sciences expertise in more detail, including our experience within Gene & Cell Therapies, please contact me (away@jigsaw-research.co.uk) or Marion Gannon (mgannon@jigsaw-research.co.uk).
Andrew Way, Oct 24
References:
- https://www.mirusbio.com/fda-approved-gene-cell-therapies/
- https://www.abpi.org.uk/publications/unlocking-access-to-future-atmps-in-the-uk